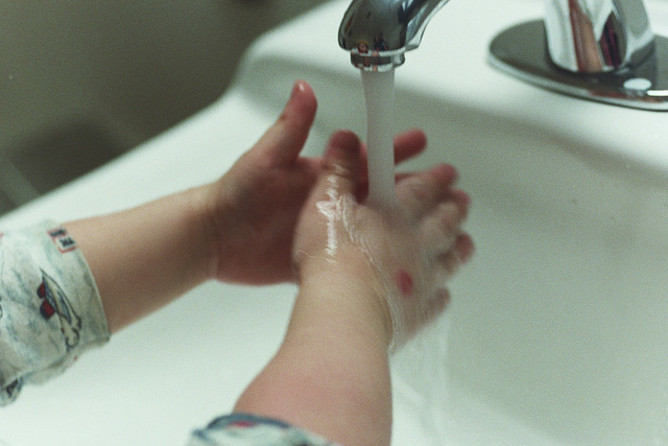
Soap washes away and eliminates viruses and germs thanks to the properties of its molecules.
These molecules are surfactants, composed of two distinct parts: a hydrophilic end attracted to water; and a hydrophobic end attracted to a virus’s fatty lipidic membrane.
Once in the presence of a virus, the hydrophobic part of the surfactant latches on to its membrane, while the hydrophilic end is attracted by the water molecules.
The result is that the fatty lipidic membrane of the virus is broken down.
The virus becomes inactive and is detached from the skin and washed away when the hands are rubbed and rinsed in water.
To be effective against viruses, hand-washing should last at least 30 seconds.
In the absence of soap and water, a santitiser may be used. A disinfectant containing 60 percent ethanol eliminates germs, but to be efficient it must be applied in sufficient quantities.
If a surface is too dirty, a sanitizer can’t reach all microbes.
Source: AFP, The Conversation
This report was produced by Mwanzo TV with the support of Code for Africa and Deutsche Welle Akademie Kenya





